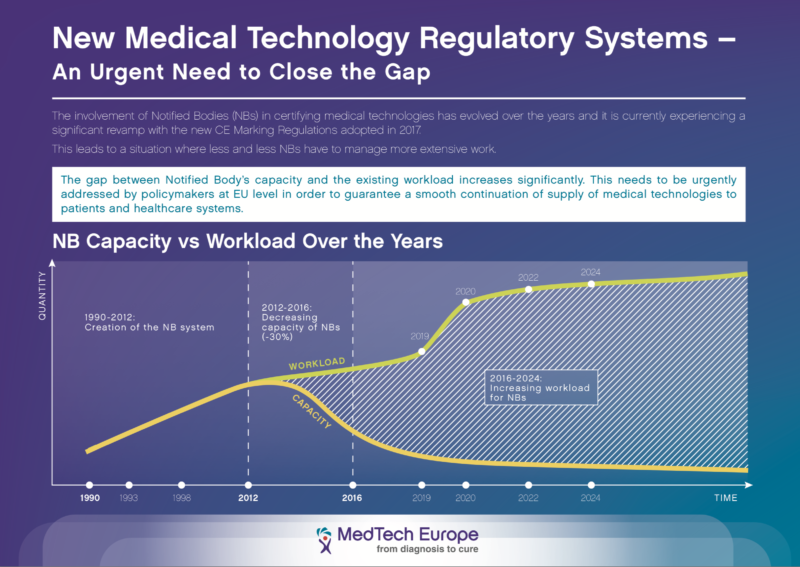
Notified bodies – Capacity vs Workload
The involvement of Notified Bodies in certifying medical technologies has evolved over the years and it is currently experiencing another significant revamp with the new CE Marking Regulations adopted in 2017.
This leads to a situation where less and less NBs have to deal with more extensive work. The gap between NBs’ capacity and the existing workload increases significantly.
This needs to be urgently addressed by policymakers in order to guarantee a smooth continuation of supply of medical technologies to patients and healthcare systems.
Posted on 31.10.2018