To address these requirements new symbols have been developed and included in ISO 15223-1:2021 Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements. Alongside, MedTech Europe has published a guidance on the use of symbols which provides translation tables for most of them as well as an Annex for symbols to be used on the implant card.
All symbols included in the ISO 15223-1: 2021 are internationally recognised symbols. In the European Union, ISO 15223-1 has been harmonised to the MDR. Therefore, in the EU these symbols do not need to be described in the information supplied with the device (as per MDR Annex I, 23. 1 h).
New symbols
All symbols included in the ISO 15223-1: 2021 are internationally recognised symbols. In the European Union, ISO 15223-1 has been harmonised to the MDR. Therefore, in the EU these symbols do not need to be described in the information supplied with the device (as per MDR Annex I, 23. 1 h)
Medical Device
Why should you add this symbol to your product label?
This symbol has been developed to meet a regulatory requirement to indicate on the label of the device that it is a medical device. Note that this symbol is only available in the ISO 15223-1: 2021, not in the OBP (Online Browsing Platform)
Contains Human Blood or Plasma Derivatives
Why should you add this symbol to your product label?
To meet a regulatory requirement, medical devices must indicate whether they contain or incorporate human blood or plasma derivatives. This symbol also helps reduce risk of misidentification, promotes patient safety and reduces the amount of training required by health care personnel.
Contains a Medicinal Substance
Why should you add this symbol to your product label?
To meet a regulatory requirement, medical devices must indicate whether they contain or incorporate a medicinal substance. This symbol also helps reduce risk of misidentification, promotes patient safety and reduces the amount of training required by healthcare personnel.
Contains hazardous substances
Why should you add this symbol to your product label?
To meet a regulatory requirement, medical devices must indicate when a medical device contains substances which are carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties. This symbol also helps reduce risk of misidentification, promotes patient safety and reduces the amount of training required by healthcare personnel.
Contains biological material of human origin
Why should you add this symbol to your product label?
To meet a regulatory requirement, medical devices must indicate whether they contain or incorporate tissues or cells, or their derivatives of human origin. This symbol also helps reduce risk of misidentification, promotes patient safety and reduces the amount of training required by health care personnel.
Contains biological material of animal origin
Why should you add this symbol to your product label?
To meet a regulatory requirement, medical devices must indicate whether they contain or incorporate tissues or cells, or their derivatives of animal origin. This symbol also helps reduce risk of misidentification, promotes patient safety and reduces the amount of training required by healthcare personnel.
Sterilised using vaporised hydrogen peroxide
Why should you add this symbol to your product label?
This symbol has been created to fulfil a regulatory requirement to indicate the method of sterilisation.
Note that this symbol is only available in the ISO 15223-1: 2021, not in the OBP.
Single Patient - multiple use
Why should you add this symbol to your product label?
This symbol has been developed to reduce the risk of misidentification and to increase patient safety. Its use is supported by reference in ISO standards and IMDRF guidance documents[1], therefore MedTech Europe recommends its use.
Translation
Why should you add this symbol to your product label?
This symbol has been developed to facilitate the indication that the original medical device information has undergone a translation which supplements or replaces the original information. This symbol should only be used by an entity that is not the manufacturer.
Repackaging
Why should you add this symbol to your product label?
This symbol has been developed to facilitate the indication that a modification to the original medical device packaging configuration has occurred. This symbol should only be used by an entity that is not the manufacturer.
Sterile barrier symbols
MDR Annex I GSPR 23.3. ‘’information on the packaging which maintains the sterile condition of a device (‘sterile packaging’) and concretely point a) of that section: ‘’an indication permitting the sterile packaging to be recognised as such’’ form the requirement for which the following four symbols have been developed.
It is for the manufacturer to select a symbol which fits their packaging configuration.
Please note that these symbols can be combined with symbols for method of sterilisation in ISO 15223-1: 2021. Please consult the standard itself and its Annex I for specific examples.
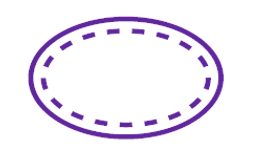
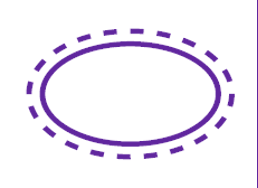
Single sterile barrier system
Double sterile barrier system
Single sterile barrier system with protective packaging inside
Single sterile barrier system with protective packaging outside
Importer
Why should you add this symbol to your product label:
To meet a regulatory requirement, medical devices must indicate the identity and address of the Importer. This symbol is useful to indicate the entity importing the medical device into the locale.
Distributor
Why should you add this symbol to your product label:
To meet a regulatory requirement, medical devices must indicate the identity and address of the Distributor. This symbol is useful to indicate the entity distributing the medical device into the locale.
The Importer and Distributor symbols have been created to indicate those entities directly on the device and can be used in both the MD and IVD sectors. It is also possible to use them together with the “Translation” and “Repackaging” symbols.
For more information on the use of these symbols, please consult ISO 15223-1: 2021 Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements. Note that useful examples are included in Annex A.
Unique device identification
Why should you add this symbol to your product label:
this symbol has been developed to facilitate the identification of the UDI carrier in cases where multiple data carriers are present.
Note that this symbol is only available in the ISO 15223-1: 2021 , not in the OBP.
Contains nano materials
Why should you add this symbol to your product label:
MDR pays increased attention to nano materials, and, while not formally requesting that specific information regarding nano materials needs to be indicated on the label, this symbol is considered useful.
Recommended symbols for use with the patient implant card
The symbols for implant card have been developed to comply with the regulatory requirements as per MDR Article 18. In some cases, symbols that had already existed in ISO have been adapted to the context of medical devices. For more information on the following symbols please consult the dedicated Annex II of MedTech Europe guidance on use of symbols for compliance with MDR. This should be consulted in conjunction with the MDCG guidance on implant cards and the ISO 15223-1: 2021 itself.
Patient Identification
Medical device
Healthcare centre or doctor
Date
Information Website
Manufacturer
Batch code or Serial number
Unique Device Identifier