IVD testing has become an indispensable tool in clinical practice. It can provide critical information at every step of the patient pathway, from prognosis, screening, diagnosis to monitoring the progression of disease, and predicting and assessing treatment responses. IVDs also play an increasing role in enabling personalised and cost-efficient healthcare delivery.
IVDs are complex interventions which can provide information on a wide range of different outcomes, depending on the contextual factors and the perspective taken. IVDs can deliver:
- Improved patient outcomes
- Better disease management by patients and healthcare professionals
- Value of knowing for individual patients
- Societal gains in early detection and the prevention of disease progression
- Public health benefits from prevention of disease spreading
- Economic savings and resource efficiencies for healthcare institutions and health systems
Essentially, IVDs provide valuable information resulting from diagnostic testing enabling users to make better informed decisions on the expected best course of action with less uncertainty.
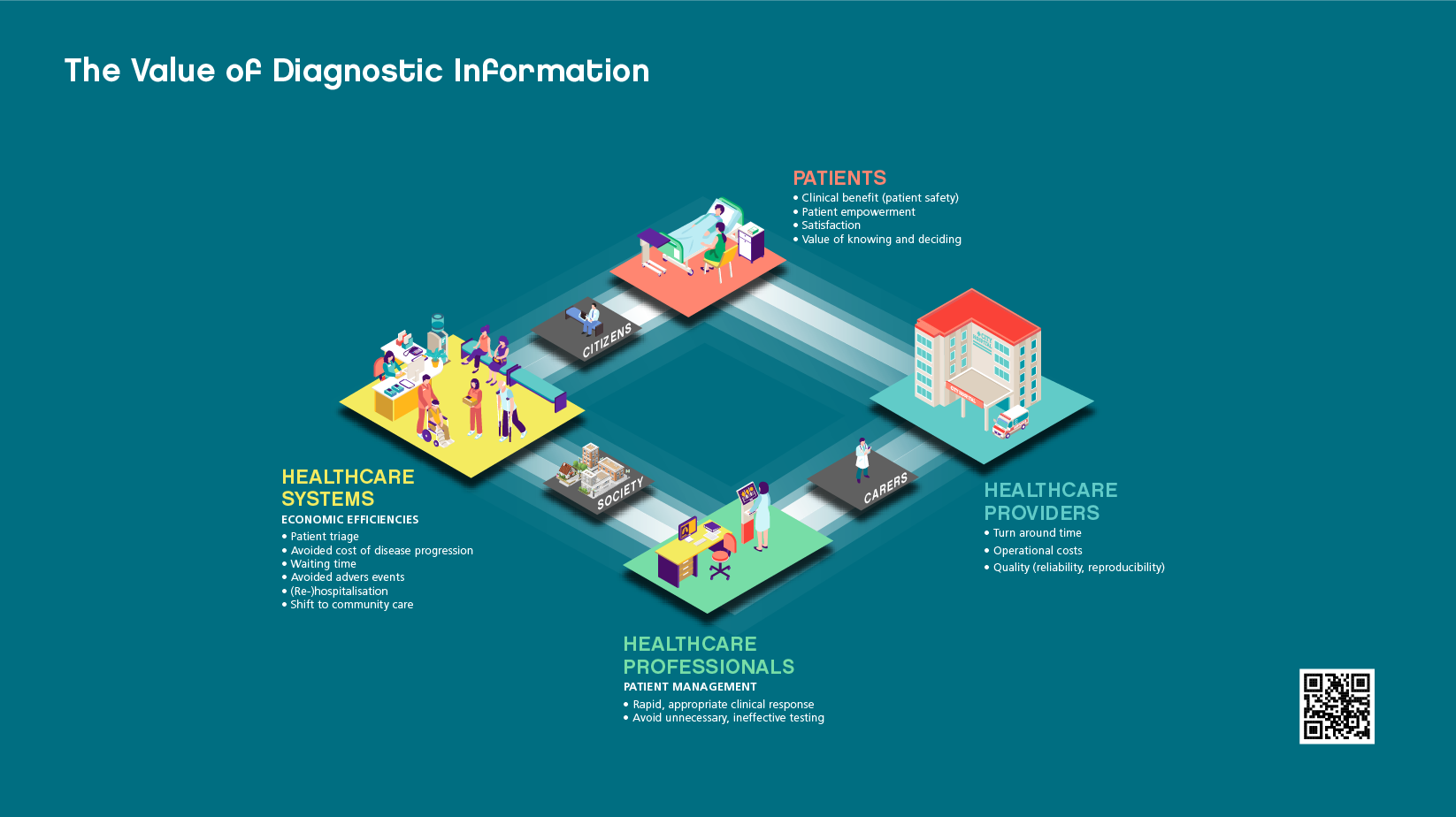
In vitro diagnostics play a vital role in achieving better patient outcomes. Results of In vitro testing influence as many as 70% of clinical decisions, while accounting for less than 2% of total healthcare expenditure. The Value of Diagnostic Information is evident and benefits stakeholders across the healthcare community. For patients, IVDs can provide clinical benefits and empowerment through knowing and deciding about their conditions. They offer healthcare professionals the opportunity to improve patient management through a faster and better-informed clinical response. For healthcare providers, IVDs allow faster turnaround time with lower operational costs. Similarly for healthcare systems they offer economic efficiencies from improved patient triage, preventing disease progression to supporting a shift from hospital care to community care.
Despite their value proposition, IVDs are seldomly rewarded based on their benefits to health systems. As such, MedTech Europe, together with a broad group of stakeholders and experts has developed a comprehensive concept for the Value of Diagnostic Information (VODI). This framework explores ways to best define, evaluate, and reward the value created from diagnostics in healthcare and how to include these value considerations in decision-making processes.
At MedTech Europe we support the idea that the full value of diagnostic information should be captured, considering what matters to patients, society and all other players involved in healthcare delivery. This can be achieved by:
- Educating patients and healthcare professionals to raise awareness of the Value of Diagnostic Information.
- Enhancing the integration of diagnostic information in healthcare systems
- Ensuring timely access to diagnostic technologies
- Utilising the full range of benefits of diagnostic information in value assessment of IVDs
- Building an enabling ecosystem that recognises and rewards the value of diagnostic information and hence incentivises future IVD innovation
If the full potential of diagnostic information is explored, MedTech Europe believes societal and individual health outcomes will improve sustainably.